“Green hydrogen” refers to hydrogen produced in a clean way powered by electricity from renewable energy. Producing green hydrogen by water electrolysis is one of the important ways to deal with the global energy crisis and achieve the goal of carbon neutrality. Industrially, water electrolysis is carried out under high current density (HCD, > 200 mA cm-2). For example, The Fuel Cells and Hydrogen Joint Undertaking in Europe (FC HJU) proposed an ambitious goal that a current density of 2500 mA cm−2 for PEM water electrolysis should be achieved by 2030. Therefore, the design and synthesis of electrocatalysts with excellent performance under high current densities, as important problems in this field, are of great significance for the industrial implementation of this technology.
Recently, Associate Professor Bilu Liu from Tsinghua Shenzhen International Graduate School (SIGS) and Professor Manish Chhowalla from Cambridge University published a review paper, summarizing several key aspects that affect the catalytic performance for high-current-density electrocatalysis, including dimensionality of catalysts, surface chemistry, electron transport path, morphology, and catalyst-electrolyte interplay, highlighting the multiscale design strategy that considers these aspects comprehensively for developing high-current-density electrocatalysts, and also putting forward out perspectives on the future directions in this emerging field.
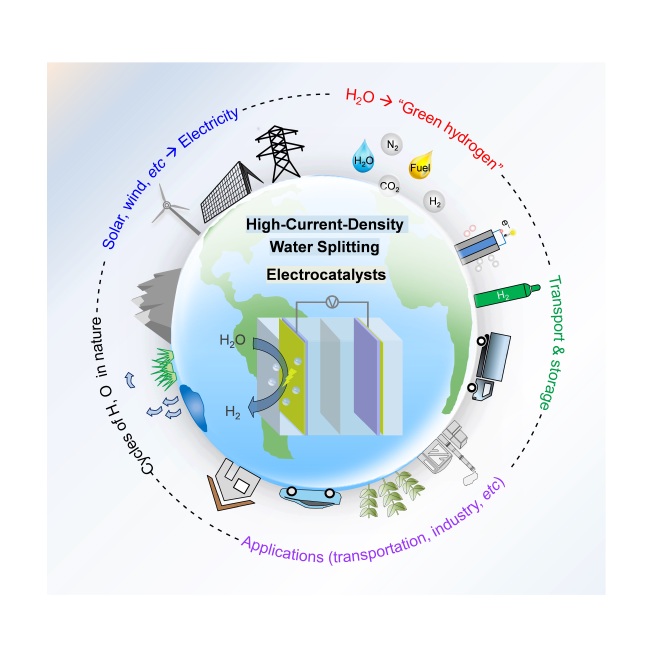
Figure 1. A schematic showing the crucial role of high-current-density (HCD) electrocatalysts for the production of “green hydrogen” by electrochemical water splitting technology coupled with renewable electricity.
Under industrial conditions, water electrolysis always works under HCDs to pursue high efficiency of hydrogen generation. There are mainly two differences between low current density and HCD conditions. First, HCD usually means that a large bias is applied to catalysts, leading to an extreme polarization condition far from the equilibrium potential. Second, the electrochemical reaction is violent and fast under HCD conditions, accompanied by the fast consumption of reactants and fast generation of products near the catalyst surface. Besides that, the difference in effects of current density on catalyst performance between the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) under HCD conditions is also noteworthy. All of the aforementioned factors will influence the overpotential and stability of the HCD catalyst.
Based on the special features of HCD water electrolysis, five key aspects that are used to engineer catalyst performance at HCDs are summarized and discussed in the review, including catalyst dimensionality, surface chemistry, morphology, electron transport path, and catalyst-electrolyte interplay.
First, the catalyst dimensionality mainly impacts the specific surface area and the electron transport path. The goal of adjusting this factor is to promote the electron transport and mass transfer process;
Second, the surface chemistry mainly affects the mass transfer process and the bond strength between the catalyst and the intermediate, which can be manipulated by interface engineering such as doping, alloy, vacancy, and heterostructure;
Third, the nano-, micro-, and macroscale structure of the catalyst will affect the number of exposed active sites and the mass transfer process, which can be adjusted by changing the size of the building blocks, channels between catalysts, and exposure of active sites;
Fourth, the electron transport path directly affects the rate and mode of electron transport, and factors such as the conductivity and the interface resistance will make differences;
Fifth, the catalyst-electrolyte interplay mainly affects the mass transfer, the bond strength between the catalyst, and the reactant or the intermediate. The main ways to regulate include changing the interfacial water, pH, or surface adsorption inside the internal Helmholtz layer.
The five aspects discussed above are used to engineer catalysts to achieve efficient HCD water splitting. The engineering of any single aspect cannot produce catalysts with a superior HCD performance, and hence a multiscale design strategy for catalysts that engineering several aspects at the same time is necessary.
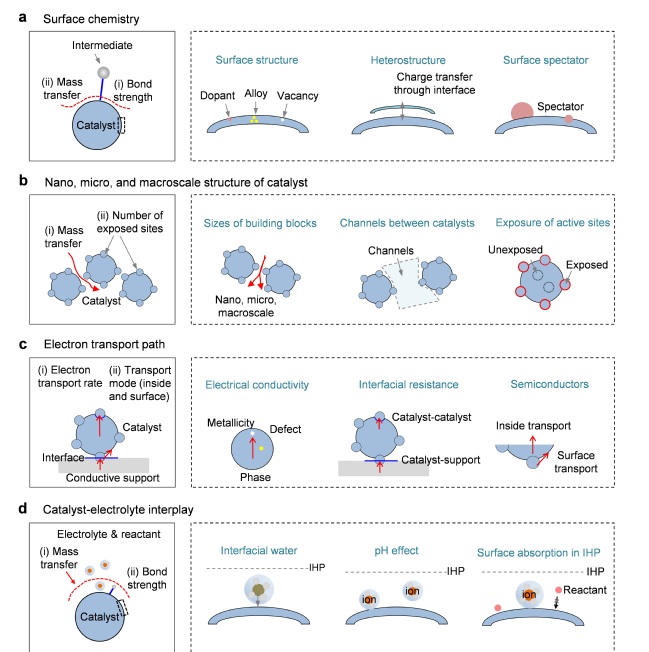
Figure 2. Schematics showing several key aspects of design of catalysts for HCD water splitting, including (a) catalyst surface chemistry, (b) catalyst morphology, (c) electron transport path, and (d) catalyst-electrolyte interplay, respectively.
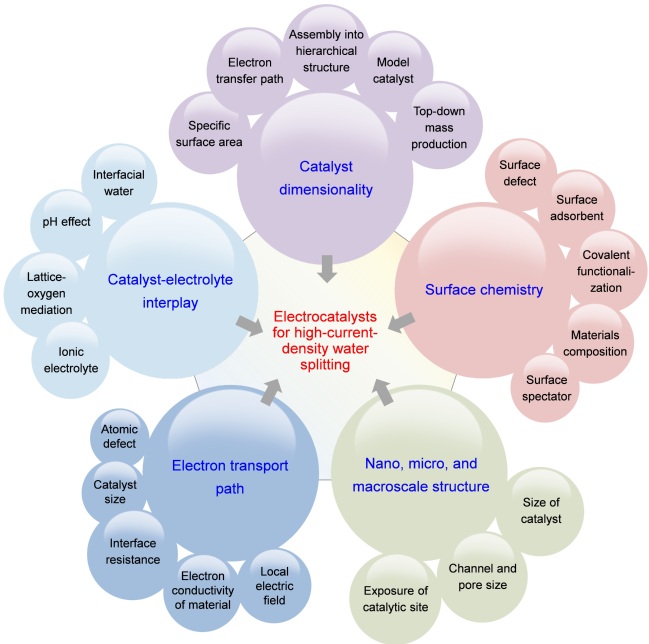
Figure 3. Summary of the five key aspects that determine electrocatalyst performance under the HCD conditions, including catalyst dimensionality, surface chemistry, morphology, electron transport path, and catalyst-electrolyte interplay.
The performance of state-of-art HCD electrocatalysts is still far from their target values. Therefore, it is still crucial to explore HCD electrocatalysts for industrial applications. The review proposed several research directions that should be pursued in the future, including in-depth understanding about electrochemical interfaces under HCD conditions, developing multiscale design strategies for HCD electrocatalysts, developing standards for evaluating catalyst performance relevant to industrial use, and taking economic considerations for the future development of HCD electrocatalysts.
This review has recently been published in the journal Advanced Materials titled “Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting.” The corresponding authors are Prof. Bilu Liu and Prof. Manish Chhowalla. The first author is Dr. Yuting Luo from SIGS (now a postdoc fellow in University of Toronto). The second author is Zhiyuan Zhang, a PhD candidate at SIGS. This work was supported by the National Natural Science Foundation of China; Guangdong Innovative and Entrepreneurial Research Team Program; Science, Technology and Innovation Commission of Shenzhen Municipality; Development and Reform Commission of Shenzhen Municipality; and the Industry and Information Technology Bureau of Shenzhen Municipality.
Link to full article:
https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202108133
Written by Xin Kang
Edited by Alena Shish & Yuan Yang


